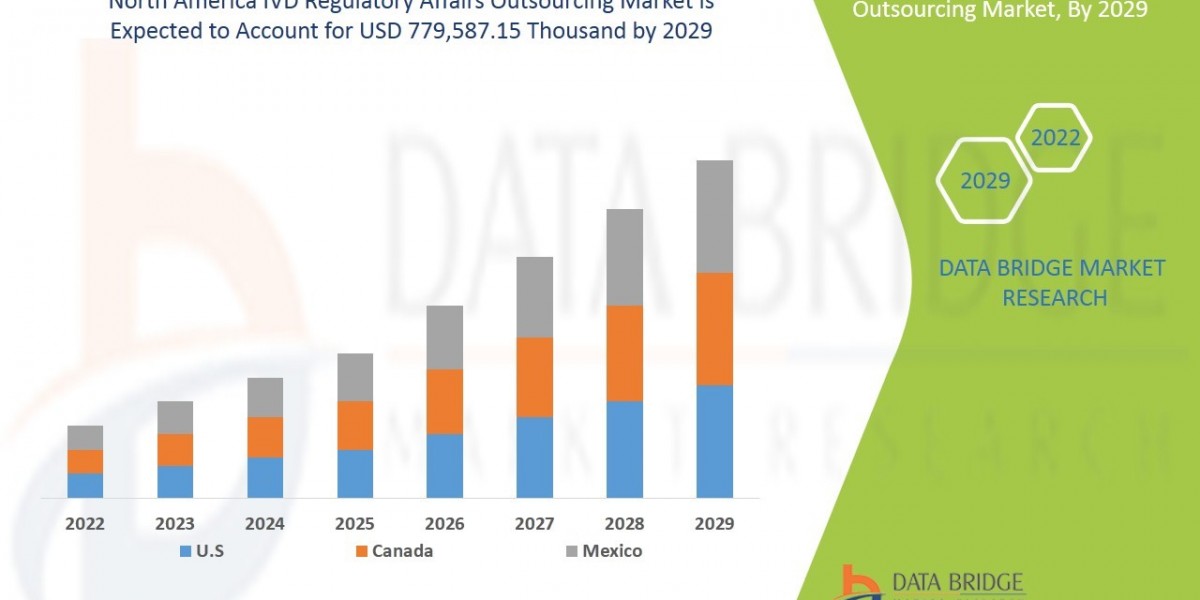
"Executive Summary North America IVD Regulatory Affairs Outsourcing Market :
North America IVD regulatory affairs outsourcing market is expected to gain market growth in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with the CAGR of 13.4% in the forecast period of 2022 to 2029 and expected to reach USD 779,587.15 thousand by 2029.
North America IVD Regulatory Affairs Outsourcing Market research report is the comprehensive analysis on the study of industry. Further, manufacturer can adjust production according to the conditions of demand which are analysed here. Analysis and discussion of important industry trends, market size, and market share estimates are revealed in the report. Additionally, the report helps the manufacturer in finding out the effectiveness of the existing channels of distribution, advertising programmes or media, selling methods and the best way of distributing the goods to the eventual consumers. The world class North America IVD Regulatory Affairs Outsourcing Market report also supports to secure economies in the distribution of products and find out the best way of approaching the potential.
By understanding and keeping into focus customer requirement, one method or combination of many steps have been employed to structure the most excellent North America IVD Regulatory Affairs Outsourcing Market research report. The report is generated with the systematic gathering and analysis of information about individuals or organizations which is conducted through social and opinion research. This global market report analyses key factors of the industry which offers precise and accurate data and information for the business growth. What is more, competitive analysis gives a clear idea about the strategies used by the major competitors in the North America IVD Regulatory Affairs Outsourcing Market that perks up their penetration in the market.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive North America IVD Regulatory Affairs Outsourcing Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/north-america-ivd-regulatory-affairs-outsourcing-market
North America IVD Regulatory Affairs Outsourcing Market Overview
**Segments**
- On the basis of service type, the North America IVD regulatory affairs outsourcing market can be segmented into in-house services and contract services. In-house services are conducted within the company's facilities by its own experts, while contract services are outsourced to third-party regulatory affairs service providers. Companies may opt for in-house services for more control over the regulatory process, while contract services offer expertise and flexibility.
- By application, the market can be divided into clinical chemistry, microbiology, hematology, genetic testing, and others. Each of these segments has specific regulatory requirements and standards that need to be adhered to for successful clearance or approval of IVD products. Regulatory affairs outsourcing companies in North America cater to these different applications and provide specialized services accordingly.
- Based on end-user, the North America IVD regulatory affairs outsourcing market includes pharmaceutical and biotechnology companies, IVD device manufacturers, research laboratories, and others. The regulatory landscape for IVD products can vary across these end-users, with pharmaceutical companies facing stringent regulations compared to research laboratories. Outsourcing regulatory affairs assists these end-users in navigating the complex regulatory environment more effectively.
**Market Players**
- Some of the key players in the North America IVD regulatory affairs outsourcing market include:
- Registrar Corp
- Emergo by UL
- Weinberg & Associates
- Qserve Group
- ProPharma Group
- dicentra
- KEYNTEC S.L.
- MAKRO CARE
- Freyr Solutions
- PharmaLex
These companies offer a range of regulatory affairs services tailored to the IVD industry in North America. With expertise in navigating regulatory requirements, these market players facilitate the approval and compliance process for IVD products, enabling clients to bring their products to market efficiently and in compliance with regulations.
The North America IVD regulatory affairs outsourcing market is witnessing steady growth driven by the increasing complexity of regulatory requirements in the IVD industry. As companies strive to bring innovative products to market while ensuring compliance with evolving regulations, the demand for specialized regulatory affairs services continues to rise. The segmentation of the market based on service type, application, and end-user reflects the diverse needs of companies operating in the IVD sector. In-house services provide companies with direct control over the regulatory process, allowing them to manage it internally, while contract services offer access to external expertise and flexibility to adapt to changing regulatory landscapes.
The segmentation of the market by application highlights the specific regulatory requirements that companies must navigate in areas such as clinical chemistry, microbiology, hematology, and genetic testing. Each of these segments has unique regulatory challenges that require specialized knowledge and experience to address effectively. Regulatory affairs outsourcing companies in North America cater to these diverse applications by offering tailored services that align with the regulatory standards governing each segment. This specialization enables companies to navigate the complex regulatory environment with precision and efficiency.
Furthermore, the segmentation of the market by end-user underscores the varying regulatory landscapes faced by pharmaceutical and biotechnology companies, IVD device manufacturers, and research laboratories. Pharmaceutical companies, in particular, are subject to stringent regulatory requirements that demand a high level of expertise and compliance. By outsourcing regulatory affairs services, companies across these end-user segments can benefit from the specialized knowledge and experience offered by regulatory affairs providers, enabling them to streamline the approval process and ensure adherence to regulatory standards.
The key players in the North America IVD regulatory affairs outsourcing market play a crucial role in facilitating regulatory compliance and market approval for IVD products. These companies offer a range of services tailored to the unique needs of the IVD industry, providing clients with the support and expertise required to navigate the regulatory landscape effectively. By leveraging the experience and knowledge of these market players, companies can accelerate the approval process for their IVD products, ensuring timely market entry and compliance with regulatory requirements.
In conclusion, the North America IVD regulatory affairs outsourcing market continues to evolve in response to the growing complexity of regulatory standards in the IVD industry. With a focus on service type, application, and end-user segmentation, regulatory affairs outsourcing companies are well-positioned to meet the diverse needs of companies operating in this sector. By partnering with key players in the market and leveraging their expertise, companies can navigate the regulatory landscape with confidence, bringing innovative IVD products to market efficiently and in compliance with regulations.The North America IVD regulatory affairs outsourcing market is characterized by a dynamic landscape driven by the increasing complexity of regulatory requirements within the industry. Companies operating in the in-vitro diagnostic sector are constantly challenged to bring innovative products to market while ensuring compliance with stringent and evolving regulations. This growing demand for specialized regulatory affairs services has led to the segmentation of the market based on service type, application, and end-user, reflecting the diverse needs of industry players.
In terms of service type segmentation, companies can choose between in-house services and contract services for managing their regulatory affairs. In-house services provide companies with direct control over the regulatory process, allowing for internal management and oversight. On the other hand, contract services offer access to external expertise and flexibility to adapt to changing regulatory landscapes, providing companies with specialized knowledge and resources to navigate complex regulatory requirements efficiently.
The segmentation of the market by application underscores the specific regulatory challenges faced by different sectors within the IVD industry, such as clinical chemistry, microbiology, hematology, and genetic testing. Each of these segments has unique regulatory requirements that necessitate specialized knowledge and experience for effective compliance. Regulatory affairs outsourcing companies in North America cater to these diverse applications by offering tailored services that align with the regulatory standards governing each segment, enabling companies to navigate the intricate regulatory environment with precision and efficiency.
Furthermore, the segmentation of the market by end-user highlights the varying regulatory landscapes faced by pharmaceutical and biotechnology companies, IVD device manufacturers, research laboratories, and other players within the industry. Pharmaceutical companies, in particular, are subject to rigorous regulatory requirements that demand a high level of expertise and compliance. By outsourcing regulatory affairs services, companies across these end-user segments can leverage the specialized knowledge and experience offered by regulatory affairs providers to streamline the approval process and ensure adherence to regulatory standards, facilitating market entry and compliance effectively.
Overall, the key players in the North America IVD regulatory affairs outsourcing market play a pivotal role in supporting companies in achieving regulatory compliance and market approval for their IVD products. These market players offer a comprehensive range of services tailored to the unique needs of the industry, providing clients with essential support and expertise to navigate the regulatory landscape effectively. By collaborating with these key players and leveraging their experience, companies can expedite the approval process for their IVD products, ensuring timely market entry and compliance with regulatory requirements.
The North America IVD Regulatory Affairs Outsourcing Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/north-america-ivd-regulatory-affairs-outsourcing-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
Answers That the Report Acknowledges:
- Market size and growth rate during forecast period
- Key factors driving the North America IVD Regulatory Affairs Outsourcing Market
- Key market trends cracking up the growth of the North America IVD Regulatory Affairs Outsourcing Market.
- Challenges to market growth
- Key vendors of North America IVD Regulatory Affairs Outsourcing Market
- Opportunities and threats faces by the existing vendors in Global North America IVD Regulatory Affairs Outsourcing Market
- Trending factors influencing the market in the geographical regions
- Strategic initiatives focusing the leading vendors
- PEST analysis of the market in the five major regions
Browse More Reports:
Global Medical Oxygen Sensors Market
Global Inferior Vena Cava (IVC) Filter Market
Global Electronic Medical Records (EMR) Market
Global Agriculture Tractor Tires Market
Global Food Processing and Handling Equipment Market
Global Anaplastic Astrocytoma Market
Asia-Pacific Surface Disinfectant Wipes Market
Global Hitter Based Hand Tools Market
North America Plant-Based Beverages Market
Middle East and Africa Plant-Based Beverages Market
North America Chlor-Alkali Market
Global Cell Harvesting Market
Europe Electronic Components Market
Global Body in White Market
Global Industrial Insulation Market
Global Veterinary Stereotactic Radiosurgery System Market
Europe Mass Spectrometry Devices Market
Global Rangefinder Market
Middle East and Africa VHF Data Exchange System (VDES) Market
Europe Influencer Marketing Platform Market
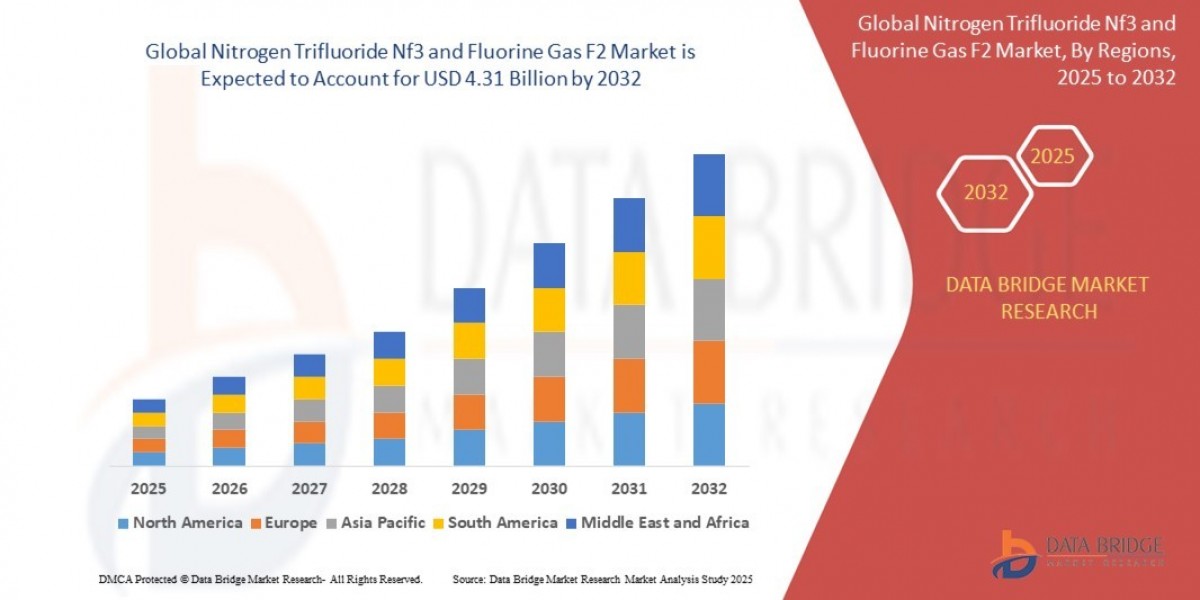
Global Nitrogen Trifluoride Nf3 and Fluorine Gas F2 Market
Global Shrink Bags Market
Global Erythema Multiforme-Stevens Johnson Syndrome Treatment Market
Global Amebiasis Treatment Market
North America Sports Apparel Market
Global Polytetrafluoroethylene (PTFE) Fabric Market
Global Buccal Drug Delivery Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com