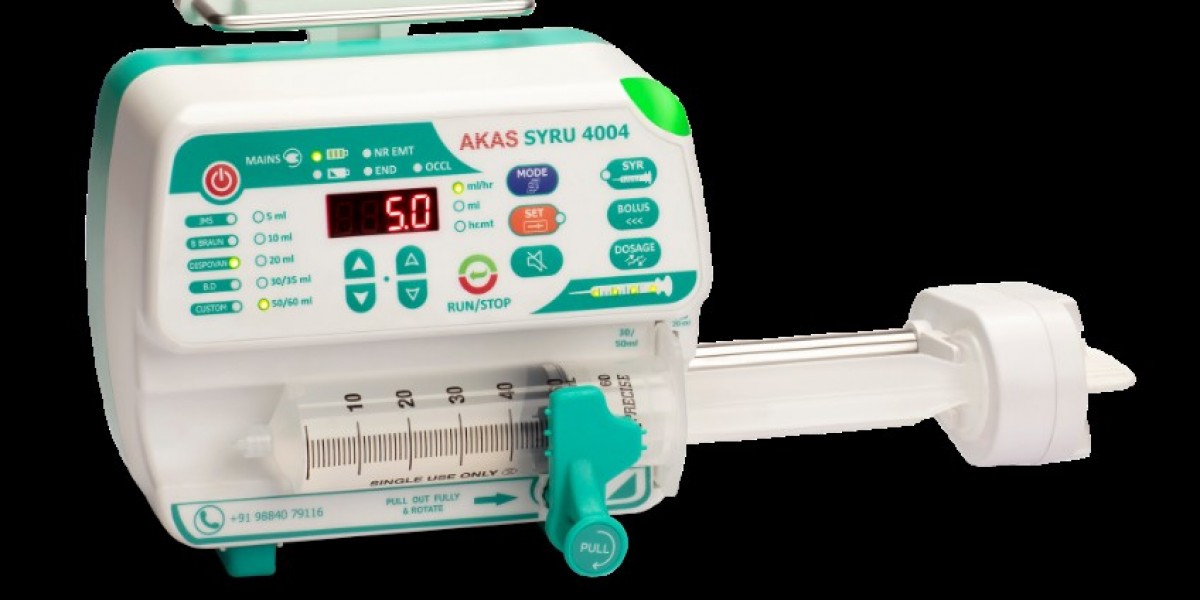
Ensuring patient safety is the foremost responsibility of hospitals and clinics. Syringe pumps play a critical role in delivering precise doses of medication, especially in intensive care, anaesthesia, and neonatal care. Selecting a syringe pump that meets rigorous safety standards is essential for protecting patients and supporting clinical staff.
Hospitals must understand the safety expectations they should demand from syringe pump manufacturers. Compliance with global and regional safety standards is not just a regulatory formality but a safeguard for accurate drug delivery, reduced errors, and overall patient well-being.
Why Safety Standards Matter in Syringe Pumps
Syringe pumps are designed to deliver controlled amounts of medication over precise intervals. Any deviation in performance can lead to medication errors, affecting patient recovery and trust in clinical care. Safety standards ensure that syringe pumps operate reliably under various conditions and reduce risks such as over-infusion, under-infusion, or device malfunction.
Reliable syringe pumps contribute to:
Accurate Drug Delivery: Maintaining precise flow rates reduces the risk of over- or under-dosing.
Enhanced Patient Safety: Compliance with safety standards prevents avoidable complications during critical care.
Operational Efficiency: Well-standardised devices require less frequent maintenance and reduce downtime.
Staff Confidence: Nurses and clinicians can focus on patient care instead of constant monitoring of device errors.
Key Safety Standards Hospitals Should Demand
When selecting syringe pumps, hospitals must look for compliance with recognised safety standards. These standards are set to ensure reliability, accuracy, and durability of the device in clinical environments. Key areas include:
ISO Certification: International standards like ISO 13485 indicate that the manufacturer maintains a quality management system for medical devices.
IEC 60601 Compliance: This standard ensures electrical safety and proper performance of medical equipment in patient care areas.
Alarm Systems and Alerts: Syringe pumps must have clear, reliable alarms for occlusion, empty syringes, or infusion errors.
Flow Accuracy Verification: Regular calibration and validation of flow rates ensure consistent drug administration.
Battery and Power Safety: Reliable backup power prevents interruption during critical infusions.
User Interface Safety: Intuitive displays and controls reduce the likelihood of human error during operation.
Considerations for Hospitals When Evaluating Manufacturers
Selecting the right syringe pump involves more than looking at device specifications. Hospitals and clinics should evaluate manufacturers based on:
Regulatory Compliance: Verify that the manufacturer follows local and international regulatory guidelines for medical devices.
After-Sales Support: Availability of service, maintenance, and technical support ensures long-term safety and reliability.
Training and Education: Manufacturers should provide thorough training for clinical staff to use syringe pumps correctly.
Product Innovation: Modern devices often include features like connectivity, real-time monitoring, and automated alerts for safer infusion.
Reputation and Experience: Established manufacturers with proven track records instill confidence in procurement decisions.
Hospitals should create a checklist of safety criteria before procurement, covering both technical specifications and manufacturer reliability.
Best Practices for Implementing Syringe Pump Safety
Once a hospital acquires syringe pumps, implementing safety protocols is crucial for optimal use. Recommended practices include:
Regular Calibration: Schedule routine verification to maintain accurate flow rates.
Periodic Maintenance Checks: Preventive maintenance reduces the risk of device failure.
Staff Training: Continuous training ensures proper use, alarm management, and emergency procedures.
Standard Operating Procedures: Clear guidelines for infusion setup, monitoring, and troubleshooting promote consistent safety.
Monitoring and Feedback: Use real-time monitoring data to detect patterns, enhance performance, and address potential risks early.
Conclusion
Patient safety and operational efficiency depend on reliable, high-quality syringe pumps. Hospitals and clinics must demand stringent safety standards, robust alarms, accurate flow controls, and comprehensive support from manufacturers. Partnering with a trusted manufacturer ensures devices meet global safety requirements while supporting clinical workflows effectively.
Akas Infusion stands out as a manufacturer of world-class drug delivery devices, including volumetric pumps, providing hospitals with reliable, precise, and safe infusion solutions that enhance patient care and clinical confidence.